3.2. Making gels: Carrageenan
Carrageenan has been used in food
as far back as the 15th century for thickening dairy products. Commercial mass
production of carrageenan gums became feasible after World War II, and now it shows up
in everything from cream cheese to dog food, where it acts as a thickener. Modernist
cuisine dishes use it for the same reason, although typically to thicken liquids into
gels in ways that we might not think of at first glance (beer gel, anyone?).
Instructions for use. Mix 0.5% to 1.5% carrageenan into room-temperature liquid. Gently stir liquid
to avoid trapping air bubbles into the gel; lumps are okay at this stage. (They’re
hard to get out unless you have a vacuum system.) Allow to rest for an hour or so;
carrageenan takes a while to rehydrate. To set carrageenan, bring to a simmer
either on a stovetop or in an oven. If you are working with a liquid that can’t be
heated, create a thicker concentration using just water, heat that, and then mix
it into your dish.Uses. Carrageenan is used to thicken foods and to control crystal growth (e.g., in
ice cream, keeping ice crystals small prevents a gritty texture). Carrageenan is
commonly used in dairy (check the ingredients on your container of heavy whipping
cream!) and water-based products, such as fast-food shakes (keeps ingredients in
suspension and enhances mouth-feel) and ice creams (prevents aggregation of ice
crystals and syneresis, the expulsion of liquid from a gel).Origin and chemistry. Derived from seaweed (such as Chondrus crispus—common
name Irish moss), carrageenan refers to a family of molecules that all share a
common shape (a linear polymer that alternates between two types of sugars). The
seaweed is sundried, treated with lye, washed, and refined into a powder.
Variations in the molecular structure of carrageenan cause different levels of
gelification, so different effects can be achieved by using different types of
carrageenan (which, helpfully, grow in different varieties of red seaweed). Kappa
carrageenan (k-carrageenan) forms a stronger brittle gel, and iota carrageenan
(i-carrageenan) forms a softer brittle gel.
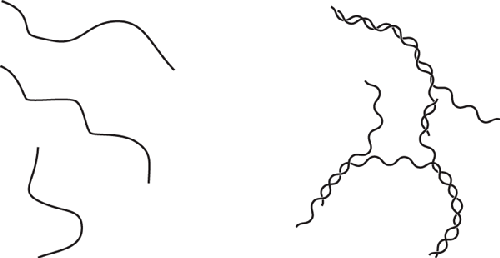
On the molecular level, carrageenan, when heated, untangles and loses
its helical structure (left); when cooled, it reforms helices that wrap around
each other and form small clusters (right). The small clusters can then form a
giant three-dimensional net that traps other molecules.
3.3. Making gels: Agar
Agar—sometimes called agar-agar—is
perhaps the oldest of all the food additives commonly used in industry, but has only
recently become known in western cuisines, mostly as a vegetarian substitute for
gelatin. First used by the Japanese in the firm, jelly-type desserts that they’re known
for, such as mizuyokan, agar has a history stretching back many
centuries.
When it comes to playing with food additives, agar is one of the simplest to work
with. You can add it to just about any liquid to create a firm gel—a 2% concentration
in, say, a cup of Earl Grey tea will make it firmer than Jell-O—and it sets quickly at
room temperature. It comes in two general varieties: flakes or powder. The powdered form
is easier to work with (just add to liquid and heat). When working with the flake
variety, presoak it for at least five minutes and make sure to cook long enough so that
it breaks down fully.
Instructions for use. Dissolve 0.5% to 2% agar by weight in cold liquid and whisk to combine. Bring
liquid to a boil. As with carrageenan, you can create a thicker concentrate and
add that to a target liquid if the target liquid can’t be boiled. Compared to
carrageenan, agar has a broader range of substances in which it will work, but it
requires a higher temperature to set.Use. Agar is a gelling agent, used in industry in lieu of gelatin in products such
as jellies, candies, cheeses, and glazes. Since agar is vegetarian, it’s a good
substitute in dishes that traditionally call for gelatin, which is derived from
animal skins and bones. Agar has a slight taste, though, so it works best with
strongly flavored dishes.Origin and chemistry. Derived from seaweed. Like carrageenan, agar is a seaweed-derived
polysaccharide used to thicken foods and create gels. When heated above 185°F /
85°C, the galactose in agar melts, and upon cooling below 90–104°F / 32–40°C it
forms a double-helix structure. (The exact gelling temperature depends on the
concentration of agar.)During gelling, the endpoints of the double helices are able to bond to each
other. Agar has a large hysteresis; that is, the temperature at which it converts
back to a gel is much lower than the temperature at which that gel melts back to a
liquid, which means that you can warm the set gel up to a moderately warm
temperature and have it remain solid.
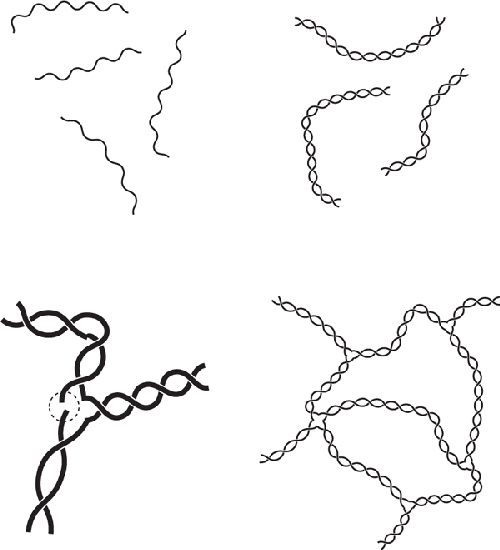
Agar at the molecular level. When heated, the molecule relaxes into a
relatively straight molecule (upper left) that upon cooling forms a double helix
with another agar molecule (center). The ends of these double helices can bond
with other agar double helices (upper right), forming a 3D mesh
(left).
Rum Screwdriver Gel
In a small mixing bowl, measure out:
8 teaspoons (40g) rum
In a saucepan, whisk to combine, and bring to a boil and hold for an additional
minute:
10 teaspoons (50g) orange juice
¼ cup (40g) sugar
1 teaspoon (2g) agar powder
Pour the hot liquid into the small mixing bowl, and stir thoroughly to combine.
Transfer mixture to a glass, ice cube tray, or other food mold and store in fridge for
30 minutes or until set.
Notes
-
Yes, these are basically rapid-setting Jell-O shots. Using agar
allows for a higher percentage of alcohol—you can gel rum by itself if
careful—but make sure to leave enough juice in for flavor. -
Play with substitutions. You can replace the rum and orange juice
with fluids such as Malibu and coconut milk.